Conservation of Charges
Description:
In our nature there are so many quantities that are conserved. We have heard of many conservation laws like conservation of mass, conservation of momentum, conservation of energy and lot more. Similarly, in nature total charge of universe is conserved.
So, conservation law says that −
We cannot destroy or create a charge. The number of total number of charges in the nature remains constant (net charge remains 0) we can only transfer it from one body to another.
Ex.: 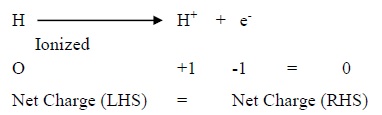
According to Einstein principle, material and energy are same. When the energy is compressed it becomes matter and when the matter is opened up it becomes energy. This is given by the relation: E=mC2
On the basis of this the principle of atomic bomb was found, i.e in atomic bomb reaction a lot of energy is produced at the cost of material (i.e electron) destruction. But we know that the electron contains charge and if the electron gets destroyed,the charge will also get destroyed, which will fail the Einstein principle of law of conservation of charge.
On the basis of this an explanation was made that there is exists an another particle called positron which gets destroyed along with the electron.
Positron
- It is the family member of nucleus.
- Its mass is same as that of electron.
- It carries a charge +e.
- It has no independent existence. It exists only inside the nucleus.
On the basis of this explanation the law of conservation of charge was modified as −
Modified Law
The charges cannot be destroyed or created individually, but it can be done in pairs. The net charge in the nature always remains zero.

