Electric Dipole - Introduction
Description:
DIPOLE −
If there are two equal and opposite charges fixed at a finite distance then the system is called Dipole.
If there are two charge with same magnitude but opposite in nature ( one positive and one negative) then, there exist a force of attraction between them due to which both the charges stick together.
The distance between their centres is a finite distance.
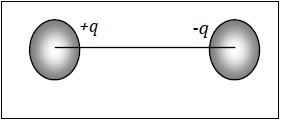
Few example of dipoles
chemical compounds like HCl, NaCl, H2SO4 etc.
H+ + Cl- → HCl

Here H+ has lost 1 electron and cl- has acquired 1 electron, so both the ions are having charge that are equal in magnitude but opposite in nature.
Here both the ions are attracted towards each other by force of attraction and stick together at a fixed finite distance to form HCl molecule. So, HCl is said to be a dipole.
Finite distance − Finite distance is a fixed distance which can neither be increased nor decreased

