Di-Electric Strength of Insulator
Description:
We know that an insulator does not allow charges to move from it. This is because the force of attraction between the electrons and the nucleus are so strong that electrons resists to leave the atom.
On applying force by an external positively charged particles, the particles will attract the electrons towards them. But if the external force is not strong enough then the electron cannot overcome the force of attraction between the electrons and the nucleus and leave its cell.
On Increasing the number of external positively charged particle the external force of attraction will increase, which will make the electron to overcome the force of attraction between the electrons and the nucleus and make the electron leave the atom.
The minimum external force which breaks the force of attraction between electrons and the nucleus and make electron move out of the atom is called breakdown field.
The maximum field strengthupto which the electron donot leave the atom thereby maintaining the property of an insulator is called dielectric strength of an insulator.
Dielectric Strength
It is the maximum strength of field, an insulator can withstand without becoming a conductor (allowing charges to pass).
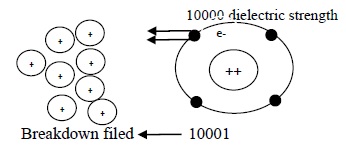
Breakdown filed
It is the minimum filed around on insulator, required to conduct charge through it, i.e. to breakdown its insulation.

