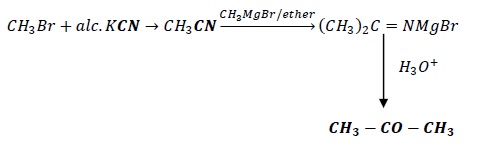Conversion Reactions (I) Problem 3
Description:
Problem
What reactions will bring about the following conversions?
1. Ethanol to but − 1 − yne
2. Benzene to biphenyl
3. Bromomethane to propanone
Solution
1. Ethanol to but − 1 − yne
Write the structure of the reactant and product.
CH3CH2OH → CH3CH2C ≡ CH
Look at the difference in the number of carbon atoms in the reactants and products.
Product has double the number of carbon atoms as compared to starting material: Wurtz Reaction.
For wurtz reaction, starting reactant should be an alkyl halide.
Ethanol is converted to ethyl iodide by reacting it with red P/I2; 2 molecules of alkyl iodide reacts with Na/ether to give butane which on reduction gives but − 1 − yne.
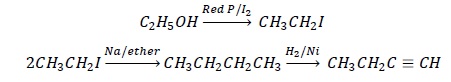
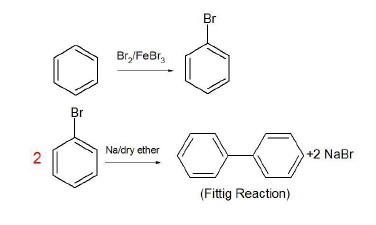
2. Benzene to biphenyl
The product has double the number of carbon atoms as compared to the reactant.
Fittig Reaction (aromatic systems).
Benzene is converted to bromobenzene and 2 molecules of bromobenzene react with Na/ether to give biphenyl.
3. Bromomethane to propanone
CH3Br → CH3COCH3
Reagent: KCN is employed whenever we need to add 1 carbon atom to the product.
Methyl bromide is converted to methyl cyanide which on reacting with methyl magnesium bromide (grignard reagent), gives an intermediate which on hydrolysis gives acetone as the final product.
Here, CH3− acts as a nucleophile and attacks the methyl cyanide.