Methods of Preparation - From Alkenes
Description:
Haloalkanes can be prepared from alkenes by adding hydrogen halides (HX) or halogens (X2) across the double bond.
Addition of HX
Alkene is converted to R − X by reacting it with HX (X = Cl, Br, I). This is an example of electrophilic addition reaction.
The reactivity of hydrogen halides towards alkenes is in the order: HI > HBr > HCl > HF.
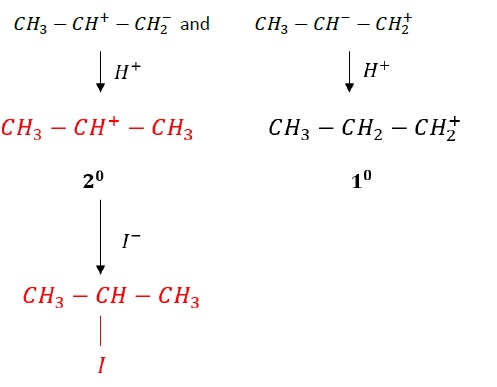
Major product obtained is determined by the addition of HX across the double bond takes place according to Markovnikov′s rule.

Example − Reaction of CH3 - CH = CH2 with HI.
Addition of X2
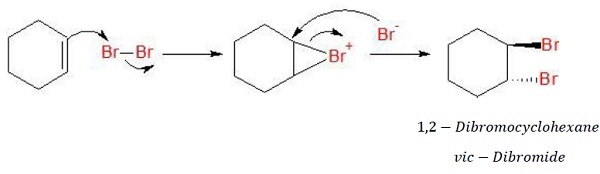
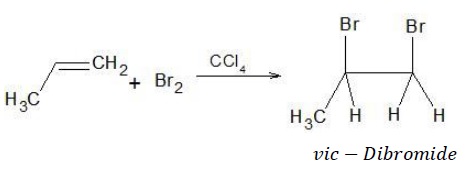
When Br2 adds to alkene in the presence of CCl4, it results in the formation of vic − dibromide.
Reaction of alkenes with Br2/CCl4, is a commonly employed test for the detection of double bond in a given compound due to the discharge of reddish brown colour during the reaction.
Mechanism of addition of halogens
The bromine molecule is polarized such as to get a partial positive charge on 1 bromine and partial negative charge on the other. Electrophilic addition takes place in such a way that a planar bromonium ion is formed. Br− now attacks the bromonium ion, opening the ring and resulting in the formation f vic − dibromide.