Reactions of Haloalkanes - Reactions with Metals
Description:
RX reacts with certain metals to give compounds containing C − Metal bonds. The resulting compounds are called organometallic compounds.
An important organometallic reagent discovered by scientist Victor Grignard was called Grignard Reagents (R − MgX).
These are obtained by the reaction of RX with Mg in the presence of ether.
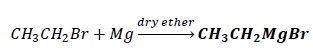
Although C − Mg bond is covalent in nature, due to the electronegativity difference between C and Mg, C − Mg bond is polar and carbon pulls electrons towards itself, resulting in δ + on Mg and δ - on C.

Mg − X bond is however ionic in nature.
Grignard reagents is highly reactive and reacts with any proton-containing substance to give hydrocarbon.
Hydrogen in H2O, R − OH, R − NH2 are sufficiently acidic to react with RMgX to give RH

Hence, anhydrous conditions (dry ether) are employed to remove any traces of water.
Wurtz Reaction
It is another example of a reaction in which haloalkanes react with metals.
In this reaction, RX reacts with Na in dry ether to give hydrocarbons with double the number of carbon atoms in RX.
2RX + 2Na → R − R + 2NaX

