IUPAC Nomenclature Problem 1
Description:
Give the IUPAC name of the given halides and classify them as primary, secondary and tertiary halides.
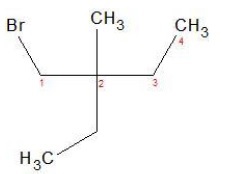
a) CH3C(C2H5)2CH2Br
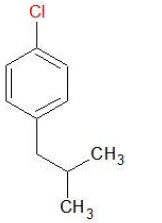
b) p − ClC6H4CH2CH(CH3)2
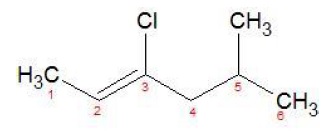
c) CH3CH = C(Cl)CH2CH(CH3)2
d) CH3CH = CH(C)Br(CH3)2
Solution
a) CH3C(C2H5)2CH2Br
IUPAC Name
1 − Bromo − 2 − ethyl − 2 − methylbutane
Primary alkyl halide.
b) p − ClC6H4CH2CH(CH3)2
IUPAC Name − 1 − Chloro − 4 − (2 − methylpropyl)benzene
Aryl Halide
c) CH3CH = C(Cl)CH2CH(CH3)2
IUPAC Name − 3 − Chloro − 5 − methylhex − 2 − ene
Vinyl Halide
- −Cl attached to sp2 carbon atom.
d) CH3CH = CH(C)Br(CH3)2
Structure −
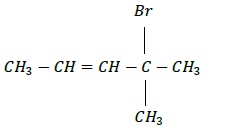
IUPAC Name − 2 − Bromo − 2 − methylpent − 3 − ene.
Allyl Halide
Carbon attached to sp3 carbon adjacent to a double bond.

