Reactions of Haloalkanes - Substitution Nucleophilic Bimolecular
Description:
Bimolecular indicates− second order kinetics, i.e., the rate depends both on the concentration of R − X and Nu−.
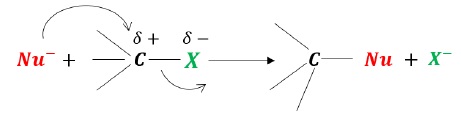
It is a one-step reaction and no intermediates are formed.
In the transition state,
Two reacting species are involved in the transition state (RDS), thus bimolecular.
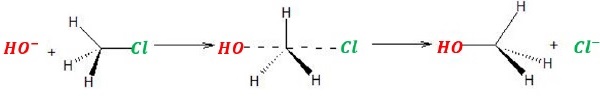
The new bond formation between HO − − − C and the bond breaking between C − − − Cl bond take place simultaneously.
Inversion of configuration takes place: back-side attack.
A complex with carbon containing 5 bonds makes it unstable.
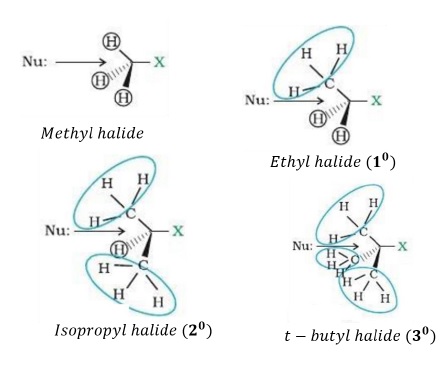
Since Nu− approaches the tetrahedral R − X, a bulky nucleophile or a bulky substrate is not favoured due to steric inhibition.
Thus, order of reactivity of R − X towards SN2 reaction is −
10 > 20 > 30
Strong nucleophile is required.
SN2 is carried out in the presence of polar aprotic solvents like DMF, DMSO favour SN2 reaction. Polar Protic solvents are not preferred for SN2 reaction because it deactivates the strong (charged) nucleophile when proton of the polar protic solvents forms hydrogen bonds with the negatively charged nucleophile. Since the reactivity of the nucleophile is decreased, it does not undergo SN2 reaction.
Reactivity of halides − R − I > R − Br > R − Cl > R − F
The steric hindrance caused by various haloalkanes can be visualized as given below: Greater the crowding around the C − X bond, more is the steric inhibition and lower is the reactivity towards SN2 reaction.
Comparison between SN1 and SN2 reaction
| SN1 | SN2 |
|---|---|
| Nucleophile strength is unimportant. | Strong nucleophile is required. |
| Formation of carbocation(2 - step) | One-step process. |
| Polar protic solvent. | Polar Aprotic solvent. |
| Require good leaving group. | Require good leaving group. |
| Retention and inversion of configuration. | Inversion of configuration. |

