Reactions of Haloalkanes - Elimination Reactions
Description:
Condition for elimination reaction: presence of β −hydrogen atom.
When RX is heated with alcoholic solution of KOH, elimination takes place.
Elimination of hydrogen from β −carbon and halogen from α −carbon.
Product is an alkene.
The reaction is also called dehydrohalogenation or β − elimination.
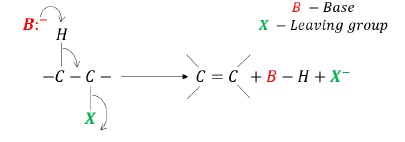
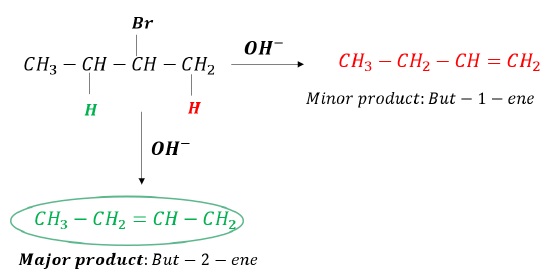
If more than 1β −hydrogen atoms are present, then multiple alkenes can be formed.
The more substituted alkene is more stable and hence the major product (Zaitsev′s product).
Greater the number of alkyl groups attached to the double bond, greater is the stability of alkene. (hyperconjugation)
For Example: In the dehydrohalogenation of 2 − Bromobutane, the major product is that alkene obtained when OH− abstracts hydrogen atom giving more substituted alkene.
Note
RX + Base → Elimination
RX + Nucleophile → Substitution (SN1 or SN2)
Which reaction gets favoured is dependent on factors such as −
- Nature of substrate (RX)
- Strength and size of the nucleophile (Nu)/base (B).
- Reaction condtions
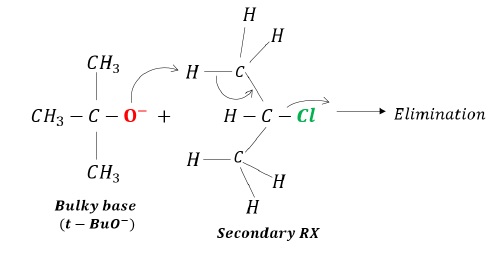
Thus, a bulkier nucleophile (t − BuO−) acts as a base and abstracts hydrogen resulting in elimination rather than act as a nucleophile as it faces steric hindrance.
For example: Steric hindrance towards substitution due to bulky base (t − BuO−)
Less bulky base (methoxide), acts as a nucleophile resulting in substitution reaction.
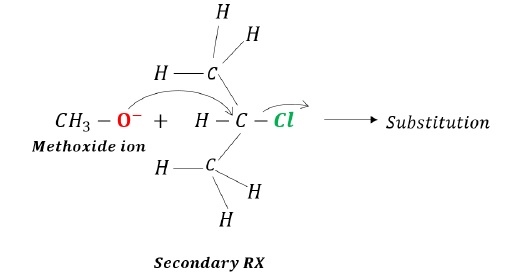
As can be seen from the above example, the substrate in both cases is same (20 alkyl halide), however, by changing the size of the attacking species, different products are obtained, one is substituted (when the attacking species is less bulky) and other is alkene (elimination, when attacking species is bulky).
To summarize, under the following situations, substitution and elimination reactions take place.
| Substrate | Substitution | Elimination |
|---|---|---|
| Primary (10) | SN2 | No |
| Secondary (20) | SN1 or SN2 Depending on strength of nucleophile | Depending on the strength of base |
| Tertiary (30) | SN1 Depending on stability of carboncation | Depending on stability of alkene |

