Haloalkanes and Haloarenes Problem 10
Description:
Problem
Primary alkyl halide C4H9Br (A) reacts with alc.KOH to give (B). This compound is then reacted with HBr to give (C) which is an isomer of (A). When (A) is reacted with sodium metal, it gives compound (D) C8H18 which is different from the compound formed when n − butylbromide is reacted with sodium.
Give the structural formula of (A) and all the reactions involved.
Solution
Given,
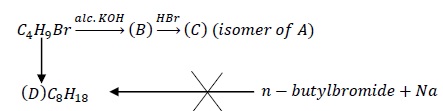
(A) is a primary alkyl halide.
Two structures of primary alkyl halide are possible from the molecular formula C4H9Br.
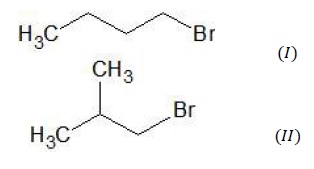
Structure (I) is n − Butylbromide while structure (II) is Isobutyl bromide. It is already given that the product (D) obtained after reacting (A) with Na/ether is not the same as the product obtained when n − Butylbromide is reacted with Na. Thus, the structure of (A) is not (I) but (II).
Thus, (A) = structure(II)
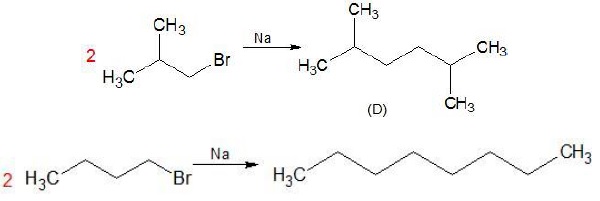
The following reaction takes place −
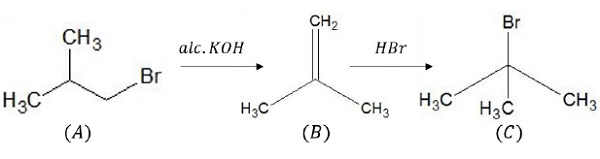
On reacting (A) with alc.KOH/Δ , elimination takes place to give alkene (B).
Alkene now reacts with HBr to give Markovnikov’s product, 30 alkyl bromide (C) which is an isomer of (A).

