Methods of Preparation - From Hydrocarbons (I)
Description:
From hydrocabons, haloalkanes and haloarenes can be prepared via −
- Free radical halogenation (haloalkanes)
- Electrophilic Substitution Reactions
- Sandmeyer reaction
Free Radical Halogenation
Since radicals are highly reactive intermediates, these are highly non-specific and results in a mixture of products. For example, free radical chlorination or bromination results in a mixture of isomeric haloalkanes. Due to difficulty in isolating a single product, it is not a preferred method.
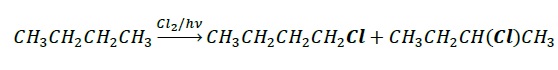
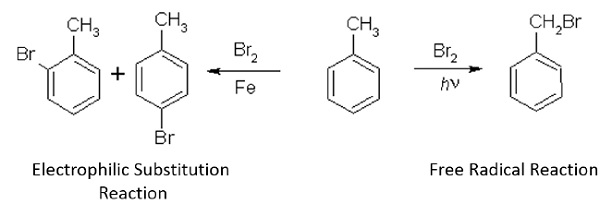
Electrophilic Substitution Reactions
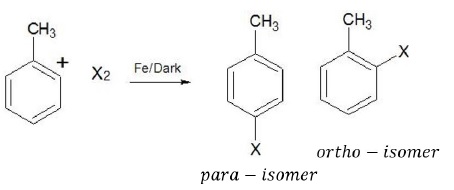
Ar − Br and Ar − Cl can be easily prepared via electrophilic substitution reactions using X2 = Cl2, Br2 in the presence of lewis acids.
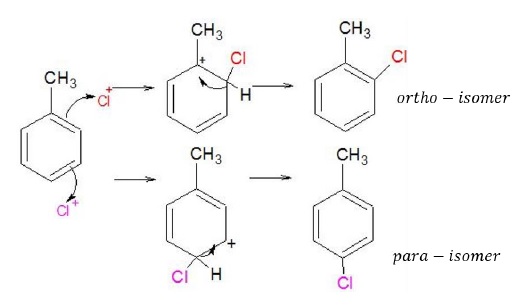
Cl2 + Fe → FeCl3 FeCl3 + Cl2 → FeCl4- + Cl+
Br2 + Fe → FeBr3 FeBr3 + Br2 → FeBr4- + Br+
- The electrophiles in these reactions are Cl+ and Br+.
- By product obtained is HCl and HBr.
Ortho and para isomers can be separated as they differ significantly in their melting points (p − isomer has higher boiling point than o − isomer).
Note: Aryl fluorides cannot be prepared by this method due to the very high reactivity of fluorine. Similarly, the reaction with iodine is reversible and requires the presence of strong oxidizing agents (Conc. HNO3 or HIO4) to oxidize HI formed during the reaction to I2 and drive the reaction in forward direction.
Problem − Why is this reaction carried out in dark?
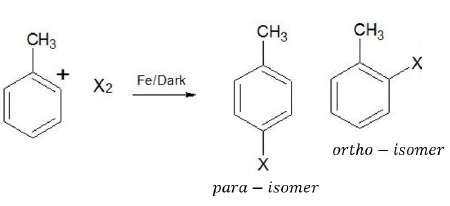
Solution − In the presence of light, halogens can undergo free radical reaction where the products obtained (benzyl bromide) are different from those obtained in electrophilic substitution reaction.