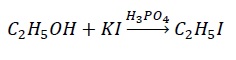Methods of Preparation - From Alcohols (II)
Description:
Reaction of alcohols with Phosphorus halides (PX5 or PX3)
Reaction with phosphorus halides replaces the −OH group of alcohols with corresponding halides.
ROH + PCl5 → RCl + POCL3 + HCl
Phosphorus tribromide and triiodide are prepared in-situ by reacting red phosphorus with bromine and iodine.

3R - OH + PX3 → 3R - X + H3PO3 (X = Cl, Br)
In the reaction of alcohols with PX3, 3 molecules of alkyl halide are obtained.
Reaction of alcohols with Thionyl chloride (SOCl2)
Reaction with SOCl2 is preferred to prepare alkyl chlorides as the by- products obtained are gaseous in nature and escapes the reaction mixture. Thus, pure alkyl halide is obtained.
CH3CH2OH + SOCl2 → CH3CH2Cl + SO2 ↑ + HCl ↑
Problem − Why H2SO4 is not preferred for use in the reaction given below?
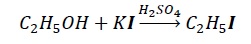
Solution − H2SO4 is a very strong acid such that it converts I− → I2. Thus, nucleophile (I−) required for the nucleophilic attack gets exhausted to I2. Hence, weak acids like H3PO4 is preferred.