Polyhalogen Compounds
Description:
Polyhalogen compounds − are those compounds containing more than 1 halogen atoms.
Dichloromethane CH2Cl2
- Used as a solvent as a paint remover, propellant in aerosols.
- Used as process solvent in the manufacture of drugs.
- Used as a metal cleaning and finishing solvent.
Harmful Effects
- Harms the central nervous system
- Exposure to higher levels causes nausea, dizziness, numbness etc.
- Direct contact with eyes burn the cornea.
Trichloromethane CHCl3
- Used as a solvent for fats, alkaloids and iodine.
- Major use is in the production of Freon refrigerant R − 22.
- Was previously used as an anesthetic as chloroform vapours depress the CNS.
Harmful Effects
Chronic exposure can lead to kidneys and lever failure.
Chloroform is stored in dark brown bottles as is it gets slowly oxidized by air to give a poisonous gas COCl2, phosgene (carbonyl chloride).
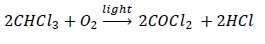
Triiodomethane CHI3
It was used previously as an antiseptic due to the liberation of free iodine.
Due to its unpleasant smell, it has now been replaced by other formulations that give iodine.
Tetrachloromethane CCl4
Carbon tetrachloride is used in the manufacture of refrigerants and propellants for aerosol cans.
Also used as feedstock in the synthesis of chlorofluorocarbons, pharmaceutical manufacturing and as a solvent.
Used as a cleaning fluid, as a degreasing agent, spot remover, and as a fire extinguisher.
Harmful Effects
Dizziness, nausea, vomiting, and permanent damage to nerve cells.
In severe cases, it can lead to stupor, coma, unconsciousness or death.
When CCl4 is released into the atmosphere, it results in depletion of ozone layer and thus increasing the exposure to harmful UV −rays leading to skin cancer, eye diseases, disruption of immune system etc.
Freons
The chlorofluorocarbon compounds of methane and ethane are called freons.
They are stable, unreactive, non-toxic, non-corrosive and easily liquefiable gases.
Freon − 12 (CCl2F2) is the most common freons used in industries.
It is manufactured from CCl4 via Swartz Reaction.
Used in aerosol propellants, refrigeration, and air conditioning.
When these freons reach the atmosphere, it initiates a radical chain reaction that affects the ozone layer balance.
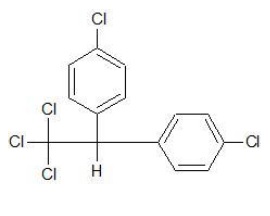
p,p′ − Dichlorodiphenyltrichloroethane (DDT)
It is the first chlorinated organic insecticides and was prepared by Paul Muller, who was awarded the Nobel Prize in Medicine and Physiology for this discovery.
It is used as an insecticide and was mainly effective against mosquito (that caused malaria) and lice (that caused typhus).
Harmful Effects
Due to its extensive use, many species of insects developed resistance to DDT.
It was also found to have high toxicity towards fish.
Due to its high chemical stability, DDT was not easily metabolized by animals and was deposited and stored in the fatty tissues.