Problem on Preparation from Alkenes
Advertisements
Description:
Problem − Give the final products obtained in each of these reactions.
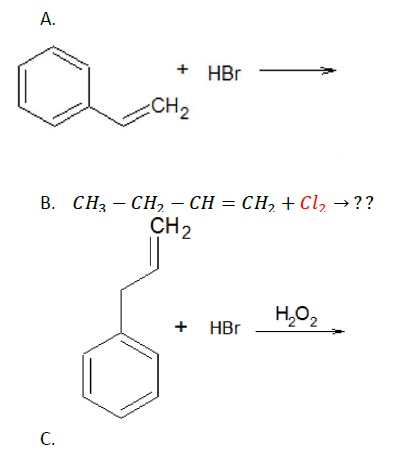
Solution −
A. Addition of HBr across double bond results in markovnikov’s product with bromine attaching to carbon with fewer number of hydrogen atoms.
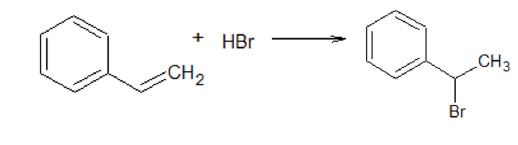
B. CH3 - CH2 - CH = CH2 + Cl2 → CH3 - CH2 - CH(Cl) - CH2Cl
Addition of halogen across double bond resulting in the formation of vicinal dihalides
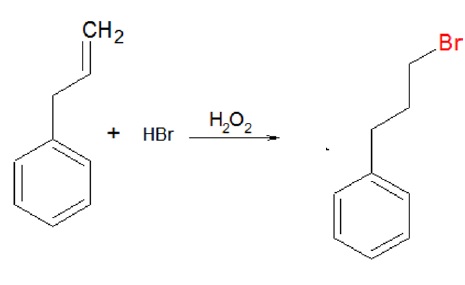
C. Addition of HBr/H2O2, results in the formation of antimarkovnikov’s product with bromine attaching to carbon atom with more number of hydrogen atoms.
Advertisements

