Reactions of Haloalkanes - Nucleophilic Substitution Reactions
Description:
The reactions of haloalkanes can be divided into −
- Nucleophilic Substitution Reactions
- SN1
- SN2
- Elimination Reactions
- Reaction with Metals
Nucleophilic Substitution Reactions are reactions in which a nucleophile attacks an electron deficient site and substitutes the X.
In alkyl halides, the more electronegative X withdraws electrons of the σ −bond towards itself polarizing the bond.
This results in a partial positive charge on the carbon atom, creating an electron deficient site.
When a nucleophile attacks this electron deficient carbon, halogen becomes leaving group and departs as halide ion.
Leaving group − I− > Br− > Cl− > F−
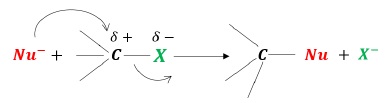
Depending on the type of nucleophiles, different products can be obtained.
| Nucleophile (Nu−) | Product (R − Nu) | Class of Compounds |
|---|---|---|
| H2O | R − OH | Alcohol |
| NaOR′ | R − O − R′ | Ether |
| NaI | R − I | Haloalkane |
| R2′ NH | R − N − R2′ | Tertiary amine |
| KCN | R − CN | Nitrile |
| AgCN | R − NC | Isonitrile |
| K − O − N = O | R − O − N = O | Alkyl nitrite |
| AgNO2 | R − NO2 | Nitroalkane |
| LiAlH4(H) | R − H | Hydrocarbon |
| R′M+ | R − R′ | Alkane |
Ambident Nucleophiles − Groups like cyanides and nitrites contain two nucleophilic centers (ambident nucleophiles).
Cɵ ≡ N ↔ : C = Nɵ
KCN − When 𝑅𝑋 reacts with KCN, C acts as nucleophile and alkyl cyanides (R − CN) are formed.
KCN is ionic in nature such that in solution K+ and CN− ions are formed where the negative charge resides on carbon atom.
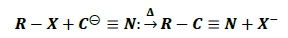
The product obtained has C − C bond which is very stable.
AgCN − When RX reacts with AgCN, N acts as nucleophile and alkyl isocyanides (R − NC) are formed.
It is because Ag − C bond is covalent in nature due to very less electronegativity difference between C and Ag.
Thus, in Ag − C ≡ N:, carbon is not nucleophilic while nitrogen acts as a nucleophile due to the presence of lone pair of electrons.
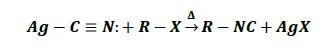
AgNO2 −
When RX is treated with Ag − O − N = O, nitroalkane is obtained.
As Ag − O bond is covalent, the lone pair of electrons on nitrogen acts as a nucleophile.
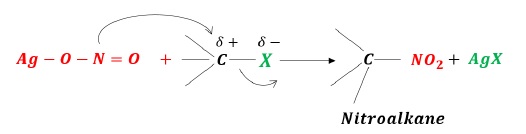
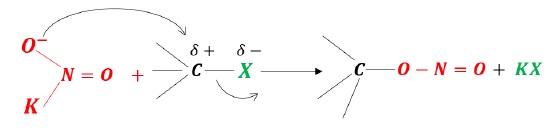
KNO2 − When RX is treated with K − NO2, alkyl nitrite is obtained.
Since K − O bond is ionic in nature, the negative charge on oxygen acts as the nucleophile.
[O − N = O]: when oxygen is the nucleophile, R − O − N = O.