Physical Properties Problem 2
Advertisements
Description:
Problem
Which of the following has the highest dipole moment?
a) CH2Cl2
b) CHCl3
c) CCl4
Solution
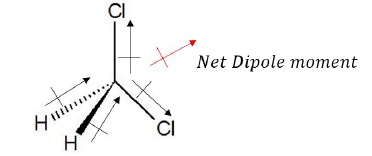
CH2Cl2 has the dipole moment.
Due to the symmetry of the structure, C − Cl bond dipole moments cancel each other, while C − H dipole moments add up and gives a net dipole moment.
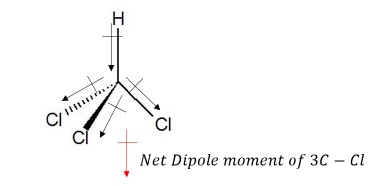
b) CHCl3
Net dipole moment of 3C − Cl bond reinforces the dipole moment of C − H such that CHCl3 has very high dipole moment.
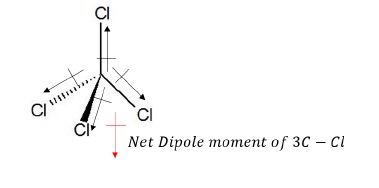
c) CCl4
Net dipole moment of 3C − Cl bond cancels the dipole moment of 3C − Cl bonds such that the net dipole moment of CCl4 molecule is zero.
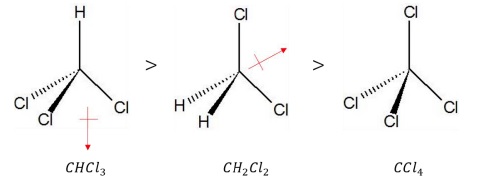
Thus, the order of dipole moment is: CHCl3 > CH2Cl2 > CCl4
Advertisements

