Haloalkanes and Haloarenes - Physical Properties
Description:
Boiling Point − Due to polarity of C − X bond and higher molecular mass, there is stronger intermolecular forces of attraction (stronger dipole-dipole and van der Waals interaction).
Boiling point order of alkyl halides: RI > RBr > RCl > RF
This is because with increase in size and molecular mass of halogen atom, boiling point also increases.
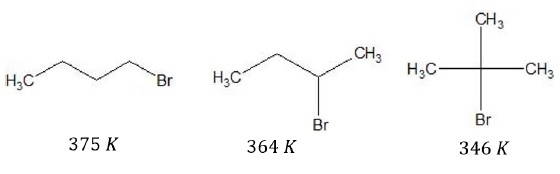
For isomeric haloalkanes, boiling point increases with decrease in branching.
More branching, lesser is the surface area, thus lesser van der Waal’s forces acting.
With increase in branching, molecule tends towards a spherical shape, such that area of contact decreases and results in weaker intermolecular forces.
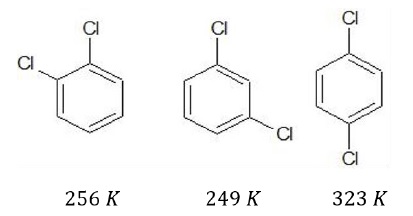
Melting point − Isomeric dihalobenzenes have similar boiling points. However, melting point differs such that para −isomer has the highest melting point.
This is because, due to greater symmetry of para −isomer, the crystal structure is highly compact resulting in greater packing efficiency. Since more number of molecules are packed in a given crystal lattice, more energy is required to break the crystal lattice.
Density − Density of haloalkanes increases with
Increase in number of carbon and halogen atoms
Atomic mass of the halogen atom
For example,
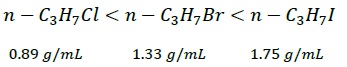
Since the number of carbon atoms are same, density increases with increase in mass of the halogen atoms.
Solubility − For dissolution, energy needs to be supplied to break the hydrogen bonds between water molecules and to overcome the attractive forces between the R − X molecules.
When alkyl halides are dissolved in water (polar solvents), Less energy is released when new bonds are formed between water molecules and R − X molecules as these bonds are not as stable as those of water molecules due to difference in polarity.
Thus, solubility of R − X in water is low.
On the contrary, alkyl halides have increased solubility in organic solvents, as the strength of intermolecular forces between R − X and organic solvents are similar to those between R − X molecules and solvent molecules.

