Reactions of Haloalkanes - Substitution Nucleophilic Unimolecular
Description:
The rate determining step is Unimolecular, i.e., the rate of the reaction follows first order kinetics and the rate depends only on the concentration of R − X.
(CH3)3C − Br + OH− → (CH3)3C − OH + Br−
It is a two-step reaction and carbocation intermediates are formed.
In the first step, C − X bond is polarized and cleaved to give carbocation.
(CH3)3C − Br ⇄ (CH3)3C+ + Br−
In the second step, nucleophile attacks the generated carbocation.
OH− + (CH3)3C+ → (CH3)3C − OH
Step I is reversible and is the rate determining step.
Stereochemistry − Since carbocation is planar, both inversion and retention of configuration takes place.
(CH3)3C − Br ⇄ (CH3)3C+ + Br−
Since the rate depends on the slowest step, which is the breaking of C − Br bond to form a carbocation, the rate depends only on the concentration of R − X and not on the nucleophile.
Thus, rate of the reaction is first order.
The energy required for breaking C − Br bond is provided via solvation of leaving group.
Polar Protic solvents (water, alcohol ) attract X and facilitates the breaking of C − X bond and formation of carbocation.
The leaving group is stabilized by protic solvent through hydrogen bonding.
(CH3)3C − Br ⇄ (CH3)3C+ + Br−
Greater the stability of carbocation, easier will be the formation of carbocation and thus, faster will be the rate of the reaction.
Thus, order of reactivity of alkyl halides towards SN1 reaction is −
30 > 20 > 10 > methyl
Hence, benzylic halide and allylic halides are more reactive towards SN1 reaction due to the formation of resonance-stabilized carbocation intermediate.
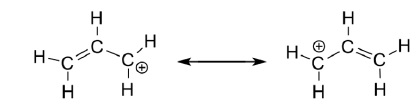
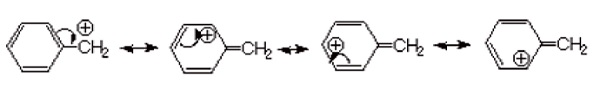
Reactivity of Halides: R − I > R − Br > R − Cl > R − F
Since the C − I bond in alkyl iodides can be broken easily and because I− is a good leaving group, nucleophile can easily attack this alkyl halide leading to substitution. However, it would be difficult for the incoming nucleophile to break the C − F bond in alkyl fluorides as the bond is very strong and F− is a poor leaving group.

