Methods of Preparation - From Alcohols (I)
Description:
Haloalkanes and Haloarenes can be prepared from −
- From alcohols (haloalkanes)
- From Hydrocarbons
- From alkenes: addition of hydrogen halides and addition of halogens.
- Halogen exchange reaction.
In this video, preparation of haloalkanes from alcohols is discussed. R − X can be obtained by reacting R − OH with suitable reagents. Reagents employed are −
Concentrated halogen acids (HX)
Phosphorus halides (PX5 Or PX3)
Thionyl chloride (SOCl2)
Reaction of alcohols with concentrated halogen acids (HX) −
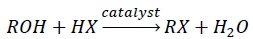
When primary and secondary alcohols react with HX, ZnCl2 is added as catalyst.
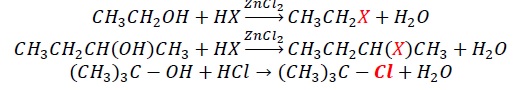
Mechanism −
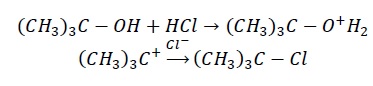
In tertiary alcohols, tertiary carbocation obtained is more stable than 20 and 10.
Reactivity of haloacids: HI > HBr > HCl > HF. Due to the weaker H − I bond, it can be broken easily to give H+ and I− ions.
The order of reactivity of alcohols with a given haloacids is −
30 > 20 > 10
As tertiary carbocations are more stable than secondary and primary carbocations and hence more reactive.
The reaction however fails to synthesize aryl halides.
The C − OH bond in phenol has partial double bond character due to delocalization of lone pair of electrons on oxygen atom with the benzene ring such that this bond cannot be easily broken by reaction with haloacids.
Ar - OH + HX → No reaction

