Problem on Elimination Vs Substitution
Advertisements
Description:
Problem
Justify the given reactions −
R − Cl + aq.KOH → R − OH
R − Cl + alc.KOH → alkene
Solution
Let the alkyl halide be CH3CH2Cl
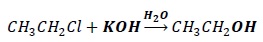
The nucleophile in the above reaction condition is OH− (in aqueous medium).
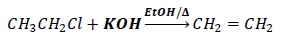
Under alcoholic conditions, the nucleophile is EtO− which is bulkier than OH−.
It would prefer to act as a base and abstract hydrogen rather than act as a nucleophile and attack the alkyl halide.
This results in elimination and not substitution.
Advertisements

