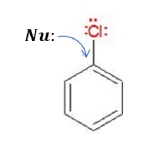
Reactions of Haloarenes - Introduction
Description:
The reactions of haloarenes can also be divided into −
- Nucleophilic Substitution Reactions
- Electrophilic Substitution Reactions
- Reaction with Metals
Haloarenes are generally unreactive towards nucleophilic substitution reactions. Under certain conditions, these undergo nucleophilic substitution reactions (which will be studied in the next video).
There are 4 main factors due to which haloarenes show decreased reactivity towards nucleophilic substitution reactions.
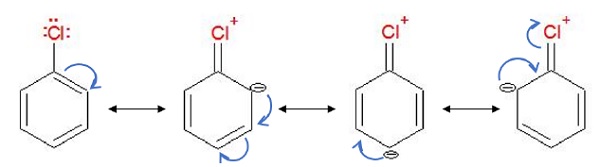
Resonance Effect
The pair of electrons on halogen atom is in conjugation with the π −electrons of the benzene ring.
Due to resonance, a partial double bond character of C − X bond which is very difficult to be cleaved by nucleophile.
Hybridization of C in C − X bond
In haloarenes, X is attached to an sp2 hybridized carbon atom.
sp2 C > s − character > sp3 C
Since sp2 C has greater s −character and is more electronegative than sp3C, it withdraws the electrons of the C − X bond towards itself.
This results in decreased bond length. Shorter the bond length, stronger the bond.
Thus, there is greater difficulty in breaking a stronger C − X bond.

Instability of Phenyl Cation
In haloarenes, when C − X bond is broken, phenyl cation and X− is formed.
Phenyl cation is highly unstable as the positive charge resides on an electronegative sp2 C atom.
It is not stabilized by any electronic displacement effects like resonance.
Due to unstable carbocation, it cannot undergo SN1 reaction.
The instability of phenyl cation can be compared to vinyl cations, both in which the carbocation resides on electronegative sp2 carbon atom. However, the stability of benzyl cation can be compared with allyl cation, both of which are stabilized by resonance.
Electronic Repulsion
An incoming nucleophile experiences repulsion from the electron-rich phenyl ring in aryl halides.