Bond Cleavage - Problem 1
Description:
Q1. a) What are the contributing structures of the compounds −
CH2 = CH - Cl
CH2 - CH = CH - CHO
b) Also indicate the relative stability of these compounds.
c) Which structure makes maximum contribution towards the resonance hybrid?
Explanation − Conditions for identifying the most stable resonance structures −
Maximum number of covalent bonds.
All the atoms satisfying octet configurations.
No charge separation.
If there is charge separation, then positive charge should reside on electropositive atom and negative charge should reside on electronegative atom.
Resonance or canonical structures of the compounds are −
a) CH2 = CH - Cl
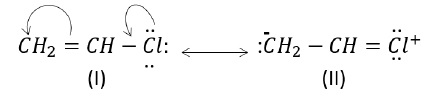
As can be seen, structure (I) is more stable than structure (II) as there is charge separation in structure (II).
b) CH2 - CH = CH - CHO
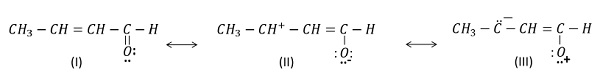
The order of stability of these structures is I > II > III
Structure (I): maximum number of covalent bonds and no charge separation. Hence, it is most stable and makes maximum contribution to the resonance hybrid.
Structure (II): is less stable than (I) due to fewer number of covalent bonds and charge separation.
Structure (III) is least stable due to fewer number of covalent bonds, charge separation and negative charge on carbon and positive charge on electronegative oxygen atom.
Q2. Which among the given compounds is more stable and why?
O2N - CH2CH2 - O- or CH3CH2O-
Explanation −
O2N − CH2 − CH2 − O− > more stable > CH3CH2O−
The presence of electron-withdrawing nitro group decreases the charge density on the small oxygen atom via −I effect. Alkyl groups (CH3CH2−) in contrast increases the electron density on oxygen atom via +I effect thereby making it highly unstable.
Q3. For each of the given bond cleavages,
- Indicate the electron flow and classify each as homolysis or heterolysis.
- Identify the reactive intermediates as free radicals, carbocation, carbanion.
a. CH3 - OCH3 → CH3O. + CH3O.
b. CH3 − CO − CH3 + OH− → CH3 − CO − CH2 − + H2O
c. (CH3)3C − Br → (CH3)3C+ + Br−
d. 
Explanation −
| Reactions | Type of Cleavage | Reactive intermediates |
|---|---|---|
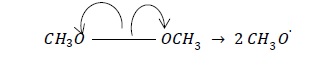 | Homolysis (Results in equally shared electron pair on both species) | Free radicals |
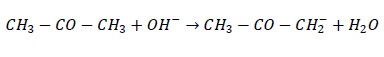 | Heterolysis OH-ion abstracts H atom from α −carbon. Electron pair is retained by carbon atom. | Carbanion |
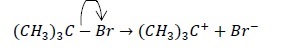 | Heterolysis Electron pair is retained by electronegative Br. | Carbocation |
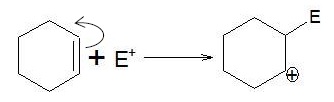 | Heterolysis π −bond shifts such that one carbon atom retains the electron pair and the other carbon atom bears positive charge. | Carbocation |
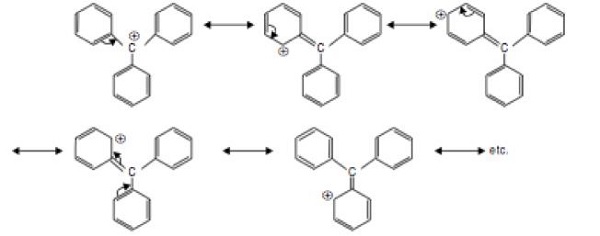
Q4. The most stable carbocation amongst the following is −
(CH3)2CH+,Ph3C+,CH3CH2+,CH2 = CH − CH2+
Explanation −
The most stable carbocation is Ph3C+. This is due to resonance stabilization of carbocation via extensive delocalization of π −bonds. More the number of resonance structures, greater dispersal of positive charge and greater stability of the carbocation.
2. The decreasing order of stability of carbanions is −
(CH3)3C− (I),(CH3)2CH− (II),CH3CH2−(III),C6H5CH2− (IV)
Explanation −
The correct order of stability of carbanions is (IV) > (III) > (II) > (I). Greater the number of alkyl groups attached to a carbanion, lesser will be the stability due to the +I effect of alkyl groups. This increases electron density on carbon and makes it less stable. Thus, 10 > 20 > 30 carbanions.
C6H5CH2− > more stable > CH3CH2−
This is because of the resonance stabilization of carbanion as the negative charge is delocalized over multiple carbon atoms and thus it is more stable.
3. The most stable free radical among the following is −
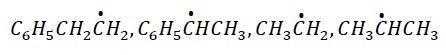
Explanation −
The most stable free radical is 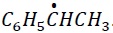 . This radical is stabilized via +I effect of −CH3 group and +R effect of C6H5 − group.
. This radical is stabilized via +I effect of −CH3 group and +R effect of C6H5 − group.
Q5. Classify the following ions/molecules as electrophile and nucleophile.
HS−,BF3,C2H5O−,(CH3)3N:,Cl+,CH3CO+,NH2−,NO2+
Explanation −
Electrophiles: Cl+,CH3CO+,NO2+,BF3
Positively charged species with sextet configuration. Hence, they can accept an electron pair thereby behaving as electrophiles. BF3 although neutral again has only 6 electrons in its valence shell and thus acts as an electrophile.
Nucleophiles: HS−,C2H5O−,NH2−,(CH3)3N:
All these species have atleast one lone pair of electrons which can be donated to electrophilic species and hence these act as nucleophiles.

