Purification Techniques - Crystallisation
Description:
Process of solidification of a pure substance from its dissolved state.
Based on the difference in the solubilities of the compound and impurities in a suitable solvent.
Most of the organic substances are only sparingly soluble in water.
Solvents like alcohol, chloroform, ether are used.
Process of crystallization involves the following steps −
Selection of solvent − A suitable solvent is the one in which
The substance dissolves on heating readily and crystallises out on cooling.
Must not be chemically reactive.
The solvent should either not dissolve the impurities or the solubility of impurities is much more than the substance to be purified. In the latter, the impurities will be left in the liquid while substance of interest crystallizes out.
Example: Ethanol purifies sugar containing common salt. Common salt does not dissolve in ethanol.
Preparing the solution −
The impure substance is powdered and heated with the solvent.
Amount of solvent is just enough to dissolve the substance on heating.
For low boiling solvents, a condenser is fitted to avoid loss of solvent on heating.
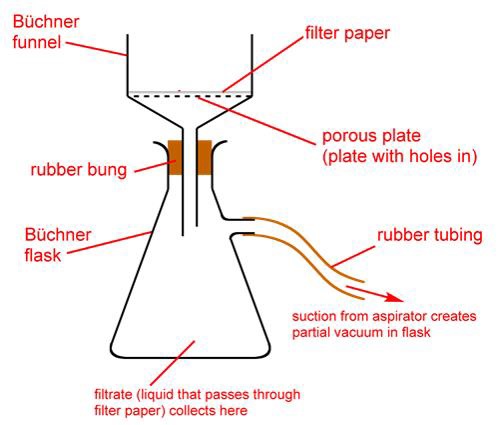
Filtration −
Saturated hot solution is filtered using filter paper placed in a glass funnel.
Crystallising −
The hot filtrate is allowed to cool slowly in a beaker.
Scratching the sides of the vessel often promotes crystallization.
Separation and drying −
Crystals are separated with the help of a Buchner funnel and suction pump.
Crystals obtained on the filter paper are washed with the pure solvent and finally
Dried over CaCl2 in a vacuum dessicator.