Electronic Displacement Effects - Problem 5
Advertisements
Description:
Problem − Arrange the following in the order of increasing stability.
CH3CH2-, HC ≡ C−, CH2 = CH-
Solution − The order of increasing stability.
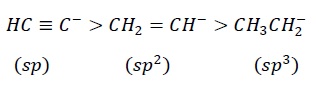
Carbanions are stabilized by electron-withdrawing groups which decrease the electron density on the carbon atom.
Electronegativity: sp > sp2 > sp3
The electron donating methyl group in CH3CH2− destabilizes the carbanion by increasing the electron density on the carbon atom via +I effect.
sp2 carbonion is more stable than Ch3CH2- however, less stable as compared to HC ≡ C− due to the higher electron withdrawing ability of sp hybridization state.
Advertisements

