Electronic Displacement Effects - Problem 1
Description:
Q1. a) What are the contributing structures of the compounds −
CH2 = CH - Cl
CH2 - CH = CH - CHO
b) Also indicate the relative stability of these compounds.
c) Which structure makes maximum contribution towards the resonance hybrid?
Explanation − Conditions for identifying the most stable resonance structures −
Maximum number of covalent bonds.
All the atoms satisfying octet configurations.
No charge separation.
If there is charge separation, then positive charge should reside on electropositive atom and negative charge should reside on electronegative atom.
Resonance or canonical structures of the compounds are −
a) CH2 = CH - Cl
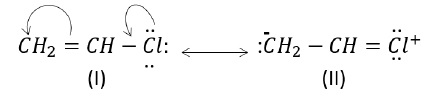
As can be seen, structure (I) is more stable than structure (II) as there is charge separation in structure (II).
b) CH2 - CH = CH - CHO
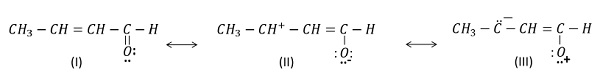
The order of stability of these structures is I > II > III
Structure (I): maximum number of covalent bonds and no charge separation. Hence, it is most stable and makes maximum contribution to the resonance hybrid.
Structure (II): is less stable than (I) due to fewer number of covalent bonds and charge separation.
Structure (III) is least stable due to fewer number of covalent bonds, charge separation and negative charge on carbon and positive charge on electronegative oxygen atom.

