Electronic Displacement Effects - Electromeric Effect
Description:
It is a temporary effect.
Exhibited by molecules containing multiple bonds and in the presence of an attacking reagent.
+E effect − π -electrons are transferred to the atom to which reagent gets attached.
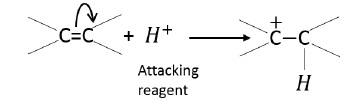
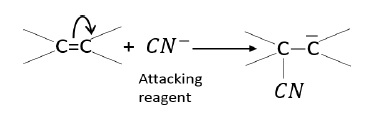
–E effect − π - electrons are transferred to that atom to which the attacking reagent does not get attached.
Example −
Identify if the mechanism of the reaction given below exhibits +E or –E effect.
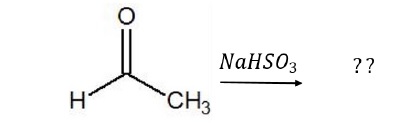
Solution − The mechanism of the reaction is as given below −
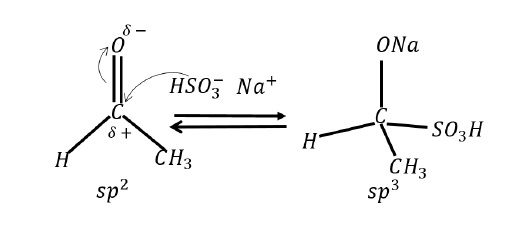
In the presence of a reagent, electronic displacements take place such that > C = O developes polarity wherein carbon and oxygen develops δ + and δ − charges respectively.
HSO3− attacks the carbonyl carbon such that the π − electrons of carbonyl carbon shifts towards oxygen.
Since π −electrons shift towards that atom to which the reagent doesn’t attach, the mechanism exhibits –E effect.
It is an equilibrium reaction and the effect vanishes as soon as the reagent is removed.