Nucleophiles & Electrophiles
Description:
Nucleophile
- Nucleophile = nucleus + philia (attacting)
- A reagent that brings an electron pair (Nu:)
- Acts as Lewis base.
- Example: carbanions
- Reaction is called nucleophilic.
Electrophile
- Electrophile = Electron + philia (attracting)
- A reagent that takes away an electron pair (E+)
- Acts as Lewis acid.
- Example: carbocations
- Reaction is called electrophilic.
| Examples | Charged ions | Neutral Molecules |
|---|---|---|
Nucleophiles | Negatively Charged OH-, CN-, R2C-, X- | H2O, NH2, R2NH |
Electrophiles | Positively charged carbocations R2C+ Group 13 halides like BCl2, BF2, AlCl3 Species like NO2+, H+, CH3CO+ | Functional groups like C = O, R - X (alkyl halides) |
During a polar organic reaction, nucleophile attacks an electron deficient site (or) electrophilic center. While electrophiles attack the electron rich center (or) nucleophilic center.
Example I
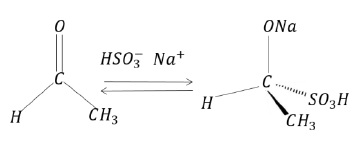
This is an example of ‘nucleophilic addition reaction’ as the nucleophile HSO3- − attacks the electrophilic center at carbonyl carbon.
Example II
CH3 − CH2 − Br + OH− → CH3CH2OH + Br−
This is an example of ‘nucleophilic substitution reaction’ where, the nucleophile OH- substitutes bromine from ethyl bromide.
Example III
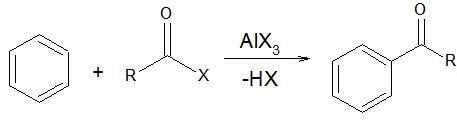
In this reaction, RCOX reacts with AlCl3 forming an electrophilic complex. The electron-rich π −bonds attack the electrophilic complex where the final product is obtained by substituting –H with the electrophilic complex. Since, the group being attached is an electrophile and substitution takes place, the reaction is called ‘electrophilic substitution reaction’.
More about these will be dealt in later chapters of organic chemistry.

