Qualitative Analysis - Test for Nitrogen
Description:
Sodalime Test
A pinch of organic compound is heated strongly with sodalime (NaOH + CaO) in a test tube.
The evolution of ammonia indicates the presence of nitrogen.
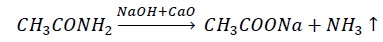
Limitation − organic compounds containing nitro and diazo groups do not liberate NH3 under the above conditions.
Lassaigne’s Test
Preparation of sodium fusion extract (alkaline).
The sodium fusion extract is boiled with iron(II)sulphate.
2-3 drops of FeCl3 solution are added and acidified with concentrated HCl.
Confirmation: formation of Prussian blue colour.
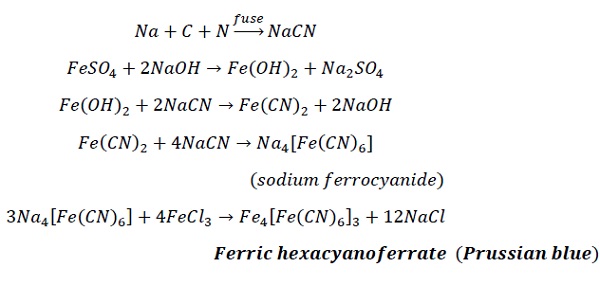
Note − Conc. HCl is added to convert ferrous hydroxide, a green precipitate to ferrous chloride which is insoluble in water, as otherwise the green precipitate may interfere with the Prussian blue colour.
Fe(OH)2 + 2HCl → FeCl2 + 2H2O
If both nitrogen and Sulphur are present, then sodium thiocyanate NaSCN is formed instead of cyanide ion NaCN such that it gives blood red colour instead of the Prussian blue colour. No free CN− ions are present as they combine with Sulphur giving thiocyanate ion.
Na + C + N + S → NaSCN
SCN- reacts with Fe3+ to give blood red colouration due to formation of Fe(SCN)2+
Fe3+ + SCN− → [Fe(SCN)]2+
If sodium fusion is carried out with excess Na metal, then thiocyanate decomposes to yield cyanide and sulphide.
NaSCN + 2Na → NaCN + Na2S

