Organic Chemistry - Carbon Hybridization
Description:
The shape, structure and reactivity of carbon compounds depend on different types of hybridization (sp3,sp2,sp). Hybridized orbitals are formed when electrons are excited from their ground state and when mixing of orbitals take place.
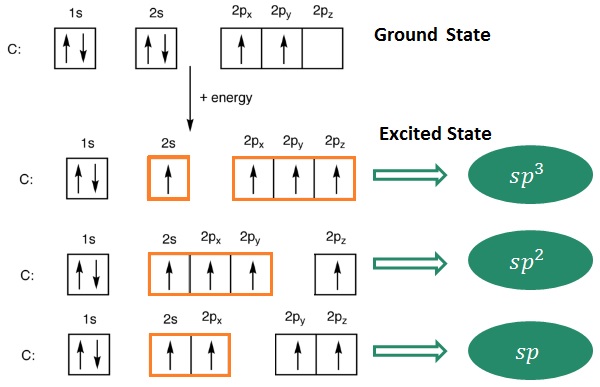
The hybridized orbitals for compounds having sp3,sp2,sp hybridization can be shown as −
CH4(sp3) | CH2 = CH2(sp2) | HC ≡ CH(sp) |
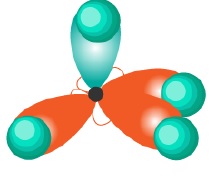 | 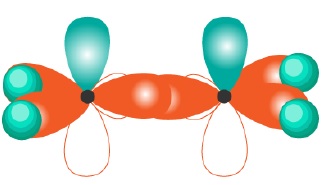 | 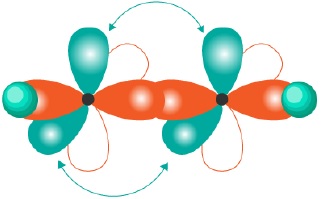 |
It can be seen that sp3 hybridization gives saturated carbon compounds while sp2 and sp hybridization results in unsaturated compounds. 1 and 2p −orbitals (py and pz) which do not participate in hybridization overlap parallely to form 1 and 2π bonds in −C = C − and −C ≡ C − systems.
| Hybridization | Structure | Bond Angle | Example |
|---|---|---|---|
| sp3 | Tetrahedral | 109.5o | CH4(methane) |
| sp2 | Trigonal Planar | 120o | CH2 = CH2(ethene) |
| sp | Linear | 180o | HC ≡ CH(acetylene) |

