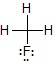Organic Chemistry - Carbon Electronic Structure
Description:
Tetravalency Carbon is a tetravalent, i.e., it has 4 valence electrons and forms 4 covalent bonds.
Covalent bonds are formed between atoms of similar electronegativity, such that sharing of electrons take place.
Lewis dot structure of carbon compounds can be drawn by −
Finding the total number of valence electrons of all atoms.
Use electron pairs to form bond between atoms.
Add the remaining electrons to atoms to fulfil octet.
For example, in CH3F,
the total no. of valence electrons of C(4) + H(1 × 3) + 7 = 14
8 electrons are required in bonding, thus remaining electrons = 6
Since C,H satisfy valence electrons, the remaining 6 e- are added to F.