Quantitative Analysis - Halogens with Example
Description:
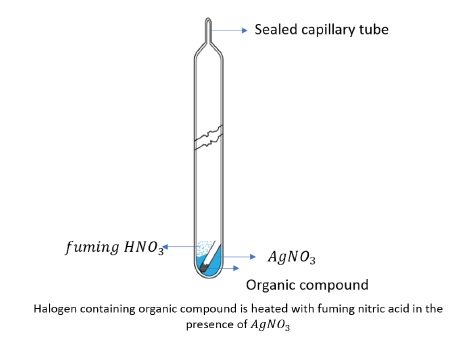
In a special type of tube called Carius tube, organic compound is heated with fuming nitric acid in the presence of silver nitrate.
Carbon and hydrogen are oxidized to CO2 and H2O.
The halogen is converted to corresponding silver halide (AgX).
It is filtered, washed, dried and weighed.
Let the mass of an organic compound = m g
Mass of AgX formed = m1 g
1 mol of AgX contains 1 mol of X
Mass of halogen in m1 g of AgX =
atomic mass of X × m1g
molecular mass of AgX
Percentage of halogen =
atomic mass of X × m1 × 100
molecular mass of AgX × m
Note − Carius method does not give satisfactory results for the estimation of iodine, as Agl is slightly soluble in HNO3.
Problem
0.20 g of organic compound is subjected to Carius Method for the estimation of halogen and resulted in 0.15 g of AgBr. Determine the percentage of bromine in the given organic compound.
Solution
Given, mass of organic compound = 0.20 g
molar mass of AgBr = 108 + 80 = 188 g/mol
188 g of AgBr contains − 80 g bromine
Thus, 0.15 g of AgBr contains =
80 × 0.15
188
= 0.063 g bromine
Percentage of bromine =
0.063 × 100
0.20
= 31.5 %

