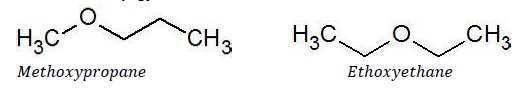Isomerism - Introduction
Description:
Isomerism refers to the phenomenon of existence of two or more compounds which have same molecular formula but different properties.
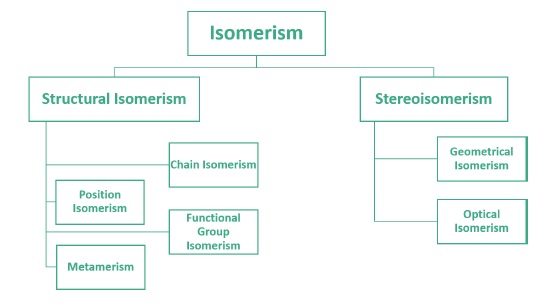
Structural Isomerism
It is observed in compounds having same molecular formula but different structures or the way in which atoms are linked to each other.
Constitutional isomers differ in their physical properties such as density, melting point, boiling point, index of refraction etc.
As the number of carbon atoms in the alkane increases, the number of constitutional isomers also increases.
For example, number of isomers in C4H10 is 2 while C5H12 has 3, C6H14 has 5 isomers and so on.
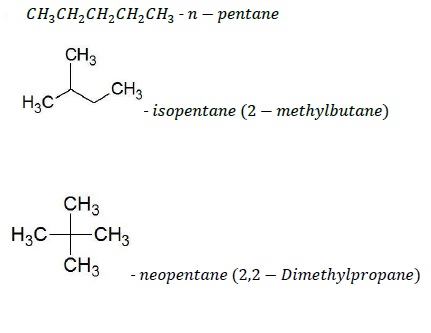
Chain Isomerism
Observed in compounds with same molecular formula but different carbon skeletons. These are also called constitutional isomers.
For example, the structures given below are the chain isomers of C5H12 (pentane).
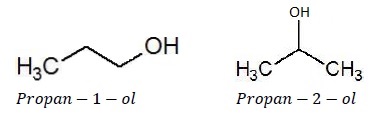
Position Isomerism
Position isomers are the compounds which differ in the position of substituent atom or functional group on the carbon skeleton.
For example, C3H8O represents two alcohols given below −
Functional Group Isomerism
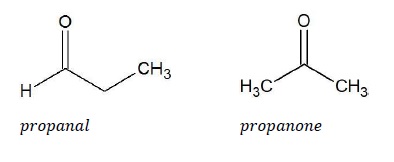
Functional isomers are those compounds which have the same molecular formula but different functional groups.
For example, C3H6O can represent an aldehyde as well as a ketone.
Metamerism
It arises due to different alkyl chains on either side of the functional group in a given molecule. For example, C4H10O represents two compounds with different alkyl chains.