Electronic Displacement Effects - Hyperconjugation
Description:
General stabilizing interaction.
Condition − The presence of α −hydrogen. (α-denotes the carbon directly attached to the functional group).
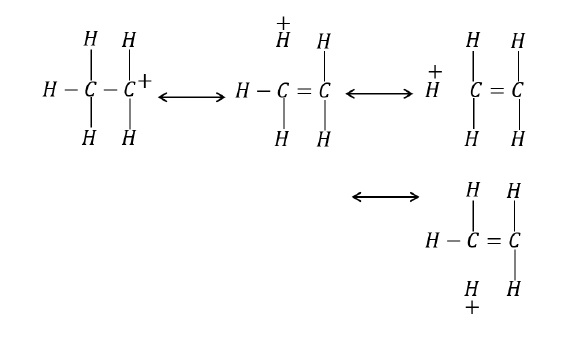
It involves delocalization of σ −electrons of C − H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p −orbital.
The σ − electrons of C − H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p − orbital.
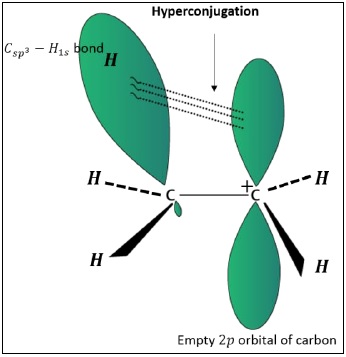
In the above figure, one of the C − H bonds of the methyl group aligns parallel to the empty p −orbitals and the electrons from the C − H bond get delocalized into the empty p −orbitals of the carbocation.
This type of overlap stabilizes the carbocation as the electron density from adjacent σ − bond helps in dispersing the positive charge.