Bond Cleavage - Problem 2
Description:
Q2. The most stable carbocation amongst the following is −
(CH3)2CH+,Ph3C+,CH3CH2+,CH2 = CH − CH2+
Explanation −
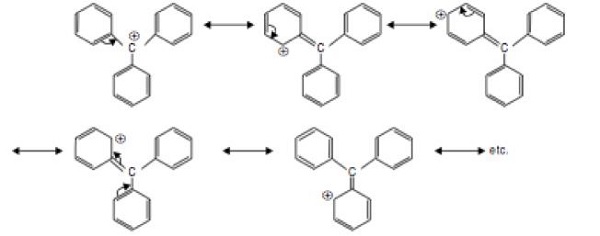
The most stable carbocation is Ph3C+. This is due to resonance stabilization of carbocation via extensive delocalization of π −bonds. More the number of resonance structures, greater dispersal of positive charge and greater stability of the carbocation.
2. The decreasing order of stability of carbanions is −
(CH3)3C− (I),(CH3)2CH− (II),CH3CH2−(III),C6H5CH2− (IV)
Explanation −
The correct order of stability of carbanions is (IV) > (III) > (II) > (I). Greater the number of alkyl groups attached to a carbanion, lesser will be the stability due to the +I effect of alkyl groups. This increases electron density on carbon and makes it less stable. Thus, 10 > 20 > 30 carbanions.
C6H5CH2− > more stable > CH3CH2−
This is because of the resonance stabilization of carbanion as the negative charge is delocalized over multiple carbon atoms and thus it is more stable.
3. The most stable free radical among the following is −
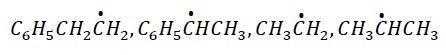
Explanation −
The most stable free radical is 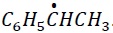 . This radical is stabilized via +I effect of −CH3 group and +R effect of C6H5 − group.
. This radical is stabilized via +I effect of −CH3 group and +R effect of C6H5 − group.

