IUPAC Nomenclature - Basic Components
Description:
Before learning about the IUPAC nomenclature, one must be familiar with the common names of a few organic compounds.
| Molecule | Common Name | Molecule | Common Name | Molecule | Common Name |
|---|---|---|---|---|---|
| CH4 | Methane | HCHO | Formaldehyde | C6H5OCH3 | Anisole |
| CH3CH2CH2CH3 | n-Butane | (CH3)2CO | Acetone | C6H5NH2 | Aniline |
| (CH3)2CHCH3 | Isobutane | CHCl3 | Choloroform | C6H5COCH3 | Acetophenone |
| (CH3)4C | Neopentane | CH3COOH | Acetic Acid | CH3OCH2CH3 | Ethyl methyl ether |
| CH3CH2CH2OH | n-Propanol | C6H6 | Benzene |
Longest chain/Parent Hydrocarbon − compound containing hydrogen and carbon.
It is the longest continuous chain containing the principal functional group defining the root name.
Other groups attached to this chain are called substituents.
Straight chain hydrocarbons − alkanes end with suffix ‘ane’ , alkenes with ‘ene’ and alkynes with ‘yne’ and prefix with the number of carbon atoms (except methane, ethane, propane and butane). For example, C5H12 is Pent-ane, C8H18 is Oct-ane, C20H42 is Icos-ane.
Branched chain hydrocarbons − in which small chains of carbon atoms (called alkyl groups) are attached to the parent hydrocarbon chain.
Alkyl group (substituent) − is derived from a saturated hydrocarbon by removing a hydrogen. Thus, −CH3 : methyl (derived from methane).
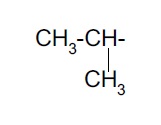 | Isopropyl- | 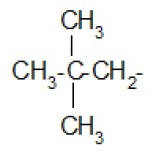 | Neopentyl- |
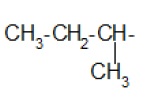 | sec-Butyl | 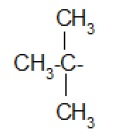 | tert-Butyl |
Functional groups − refers to an atom or group of atoms that is responsible for the characteristic chemical properties of organic compounds. For example, compounds containing –OH functional groups are alcohols while those with –COOH groups are called carboxylic acids.
A compound may contain multiple or single functional groups.
If multiple functional groups are present, then the principal functional group is the highest priority functional group and is chosen according to the order of decreasing priority.
−COOH > −SO𝟑H > −COOR > −COCl > −CONH𝟐 > −CN > −HC = O > C = O > OH > SH > NH𝟐 > C = C > −C ≡ C−> −R > −OR > −F,Cl,Br,I > −NO𝟐
If only a single functional group is present, then that becomes the principal functional group and determines the root name.
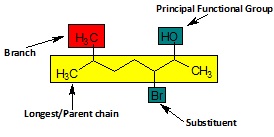
Numbering − Defines the position of principal functional group and substituents.
Numbering is done on the longest chain such that the principal functional group receives the lowest number.
If there is no functional group, then numbering is done such that branched carbon gets the lowest possible numbers.
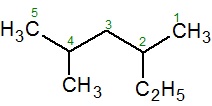
If there is a tie, then the method that gives the lower number at the first point of difference is used. For example, in the given hexane molecule, the correct naming is 2,4 − Dimethylhexane is correct and not 3,5 − Dimethylhexane.
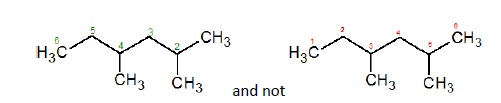
If there is no first point difference, then numbers are assigned in alphabetical order. For example, the name of the given compound is 2 − Ethyl − 4 − methylpentane and not 4−Ethyl − 2 − methylpentane.