Electronic Displacement Effects - Problem 7
Description:
Problem − Write structures of various carbocations that can be obtained from 2 − methylbutane.
Arrange them in the order of increasing stability.
Solution − 2 - methylbutane (CH3 − CH(CH3) − CH2CH3)has the following possible carbocations −
| a. | 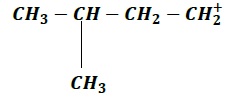 | b. | 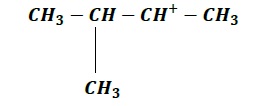 |
| c. | 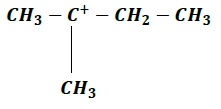 | d. | 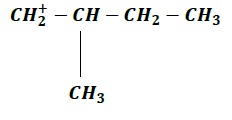 |
Order of increasing stability: c > b > d > a
Note − (a) & (d) are both primary carbocations, however (d) > more stable > (a) because −
In (d), electron donating methyl group is at the α −position while
In (a), the electron donating methyl group is at the β −position.
Electron-donating groups stabilize carbocations via +𝐼 effect and since inductive effect decreases with increase in distance, (d) is more stable than (a) due to the closer proximity of methyl group is (d) as compared to (a).

