Purification Techniques - Chromatography
Description:
It used to
- separate mixtures into their components.
- Purify compounds.
- To test the purity of compounds.
The mixture of compounds is applied to a stationary phase (solid or a liquid). A pure solvent, mixture of solvents or gas (mobile phase) is allowed to move through the stationary phase. On the basis of affinity of compounds to the adsorbent or the mobile phase, the mixture gets separated from one another.
Chromatography can be of two types −
Adsorption Chromatography
Different compounds get adsorbed to the adsorbent to different degrees.
Commonly used adsorbents are: silica gel and alumina.
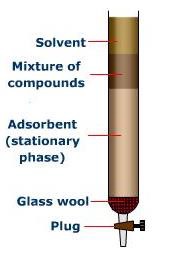
Column chromatography
Involves separation of a mixture over a column of adsorbent (stationary phase).
The mixture of compound is placed on top of the column.
It is eluted through the column using a suitable liquid, liquid mixture or gas.
Depending on the degree of affinity of the compounds to the adsorbent, they are adsorbed to adsorbent until complete separation takes place.
The affinity is usually based on factors such as polarity.
The most readily adsorbed components are retained at the top of the column.
Thin-Layer Chromatography
Here mixture is separated over a thin layer of adsorbent coated on a glass plate.
The mixture to be separated is placed as a small spot (2cm above one end) on the tlc plate.
The plate is then placed in a closed jar containing a suitable eluant.
As the eluant rises up the plate, the components of the mixture get separated and the relative adsorption of each component is given by Rf or retention factor.
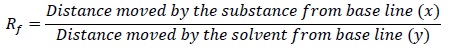
The spots of colourless compounds can be detected by putting the the plate under UV light or by immersing in a jar containing few crystals of iodine.
Other reagents to detect the spots in ninhydrin for the detection of aminoacids.
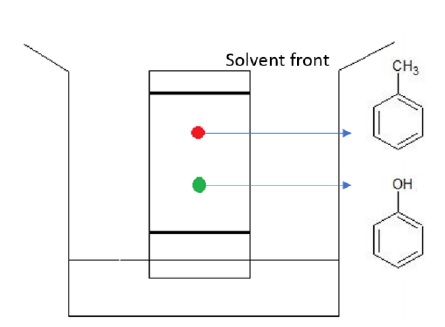
In this TLC plate, phenol is retained before toluene as phenol interacts with the adsorbent silica through strong hydrogen bonds and thus retained quickly as the eluant rises up. Toluene however, has no such strong interaction with the adsorbent and gets retained later and has thus been eluted further away.
Partition Chromatography
It is based on the partitioning of components of mixture between stationary and mobile phase.
It is an example of liquid-liquid chromatography while adsorption chromatography is based on solid-liquid chromatography.
Example: Paper chromatography.
A special quality paper known as chromatography paper is used. Paper is mainly cellulose however, water trapped in the paper of paper chromatography acts as stationary phase.
Mobile phase is a liquid mixture of two or more substances with water as one of the components.
A spot of the mixture is placed at the bottom of the chromatography paper and is suspended in a suitable solvent or a mixture of solvents.
Solvent rises up by capillary action and the paper selectively retains different components according to their differing partition in two phases.
When solvent reaches the top end, the paper is taken out and allowed to dry. The paper strip so developed is called chromatogram.
The colourless spots can be observed either by viewing under UV light or by the use of an appropriate spray reagent.

