Qualitative Analysis - Problem 1
Description:
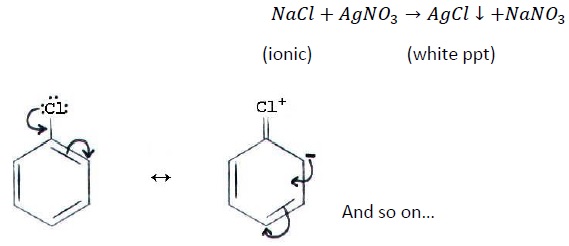
Statement I − When silver nitrate is added to chlorobenzene, white precipitate is formed.
Statement II − C − Cl bond in chlorobenzene is ionisable.
A. Statements I and II are true and II is the correct reason for I.
B. Statement I is true but II is not the correct explanation to I.
C. Statement I is false but II is true.
D. Statements I and II are both false.
Solution −
The correct option is: (D)
Explanation −
Both statements are false.
When silver nitrate solution is added to chlorobenzene, “No White PPT” is formed.
It is because, C − Cl bond in chlorobenzene is not ionisable due to the delocalization of lone pair of electrons of chlorine with the benzene ring giving the C − Cl bond a partial double bond character. Thus, it is very difficult to ionize this bond.