Organic Chemistry - Hybridization Examples
Advertisements
Description:
Example 1
Identify the number of sp3,sp2,sp carbon atoms in the given molecule.
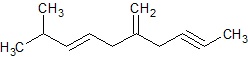
Ans − 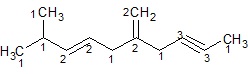
All 1’s − sp3 hybrid carbon atoms (6)
All 2’s − sp2 hybrid carbon atoms (4)
All 3’s − sp hybrid carbon atoms (2)
Example 2
What are the hybridization states involved in the given compound?
CH2 = C = CH2
Ans − Number of σ − bonds will give the hybridization. For example, 4σ bonds give sp3 hybridization, 3σ bonds give sp2 while 2σ bonds give sp hybridization.
CH2 = C = CH2
(sp2) (sp) (sp2)
Advertisements

