Quantitative Analysis - Nitrogen by Dumas Method
Description:
The two available methods for the estimation of nitrogen are: (i) Dumas Method and (ii) Kjeldahl’s method.
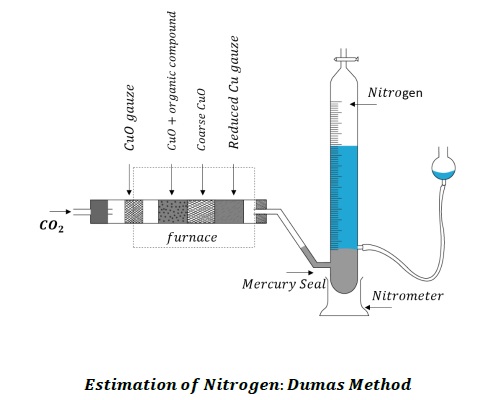
1. Dumas Method − When nitrogen containing organic compound is heated with excess copper oxide in an atmosphere of CO2, free nitrogen is obtained in addition to CO2 and water. Chemical reactions are −
C + 2CuO → CO2 + 2Cu
2H + CuO → H2O + Cu
Nitrogen + CuO → N2 + oxides of nitrogen
Any oxides of nitrogen formed during the reaction are reduced to N2 by passing it over heated copper gauze.
Oxides of nitrogen + Cu → N2 + CuO
The mixture of gases is collected over KOH solution that absorbs CO2.
N2 is collected in the upper part of the graduated tube.
The net reaction can be given as −
CxHyNz + (2x +
y
2
)CuO → xCO2 +
y
2
H2O +
z
2
N2 + (2x +
y
2
)Cu
For example,
Let the mass of organic compound = m g
Volume of nitrogen collected = v cm3
Atmospheric pressure = P mm
Room temperature = T K
Pressure of water vapour at T K (aqueous tension) = p mm
Thus, pressure of dry nitrogen = P - p mm
I. To reduce the volume of nitrogen to STP
V1 = v cm3 , V2 = ?
P1 = (P − p)mm, P2 = 760 mm
T1 = T K, T2 = 273 K
P1V1
T1
=
P2V2
T2
V2 =
(P - p)V1 x 273
760 x T1
II. Percentage of nitrogen
22400 cm3 N2 at STP weighs = 28 g
V2 cm3 of N2 at STP weighs =
28V2
22400
g
Amount of nitrogen present in ′m’ g of compound =
28 x V2
22400 x m
Percentage of N2 =
28 x VN2(STP) x 100
22400 x m

