Purification Techniques - Distillation
Description:
It is a process of conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling.
Used for the purification of liquids which boil without decomposing.
Impure liquid is boiled and vapours formed are condensed to obtain pure liquid.
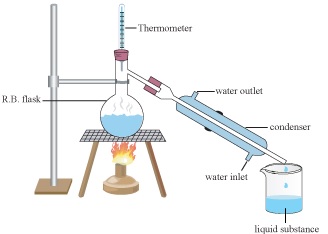
Simple Distillation
Separates volatile liquids from non-volatile impurities and liquids differing sufficiently in their boiling points.
In a mixture of liquids, the liquid with lower boiling point evaporates and the vapours are condensed and collected.
The vapours of higher boiling point are obtained later and collected separately.
For example, mixture of chloroform (B.P 334K) and aniline (B.P 457K) can be separated by distillation.
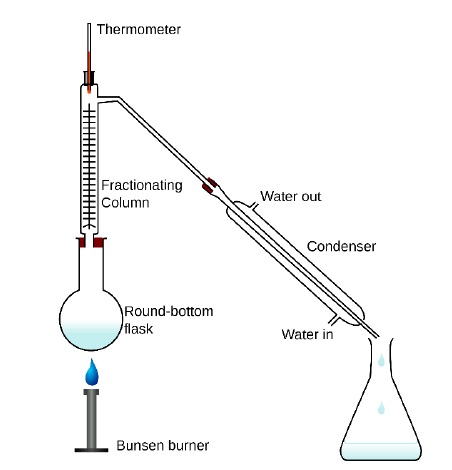
Fractional Distillation
Used to separate two or more liquids which do not differ much in their boiling points (B.P < 300C).
In this technique, the vapours of the liquid are passed through a fractionating column before condensation.
Fractionating column provides obstructions to the passage of vapours upwards and to the liquid downwards.
The vapours of the liquids with higher boiling point condense before the vapours of liquid with lower boiling point.
The vapors in the fractionating column become increasingly rich with more volatile substance.
Thus, on reaching the top of the column, vapours become pure in low boiling component and pass through the condenser and pure liquid is collected in the receiver.
After a series of successive distillations, the remaining liquid in the distillation flask gets enriched in high boiling component.
Each successive condensation and vaporization unit in the fractionating column is called a theoretical plate.
Used: to separate different fractions of crude oil in petroleum industry.
Distillation under reduced pressure
Liquid boils when its vapour pressure becomes equal to the external pressure or the applied pressure.
If the applied pressure decreases, the same liquid now boils at a lower temperature.
Thus, it is used to purify liquids having high boiling points and those which decompose at or below their boiling points.
Under reduced pressure, the liquid will boil at a low temperature and the temperature of decomposition will not be reached.
For example: glycerol boils at 563K where it also gets decomposed. If pressure is reduced to 12mm, it boils at 453K without decomposing.
Finds application in sugar industry and in soap industry to separate glycerol from spent-lye.
Steam Distillation
Used to separate substances which are steam volatile and immiscible with water.
In this method, steam is passed through a heated flask containing liquid to be distilled.
The liquid boils when partial pressure of both the liquid (p1) and water (p2) becomes equal to the atmospheric pressure (p = p1 + p2).
The temperature at which the mixture boils is lower than the boiling point of both the liquid and water.
Since p1 < p, organic liquid vaporizes before reaching the boiling point of the liquid mixture.
The mixture of steam and volatile organic compound is condensed and collected.
The compound is then separated from water using separating funnel.
For example, a mixture of aniline (B.P:453K) and water (B.P:373K), under atmospheric pressure boils at 371K.
At this temperature, vapour pressure of water is 717mm and that of aniline is 43mm such that the total vapour pressure is equal to 760mm.
Thus, if one of the substances in the mixture is water and other a water insoluble substance, the mixture will boil close to but below 373 K.
Example: separation of aniline from aniline-water mixture.

