Homolytic Cleavage - Free Radicals
Description:
Organic reactions which proceed via homolytic cleavage are called non-polar or free radical reactions.
The bond is broken such that the electron pair in a covalent bond is shared equally by both atoms.
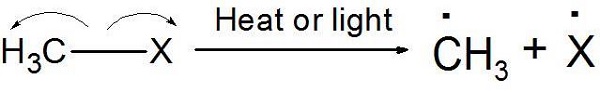
Movement of a single electron takes place. Not electron pair.
Results in neutral species with 1 unpaired electron called free radicals.
Contains a pz orbital with 1e - and has sp3 hybridization and slightly pyramidal geometry. The inversion barrier is very low such that interconversion between the two forms is rapid at room temperature.
Geometry of carbocations, carbanions and free radicals can be compared as given below −
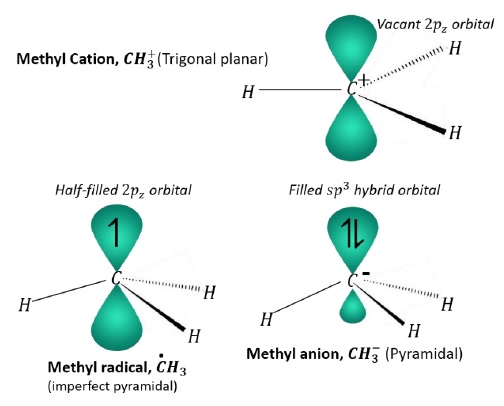
Carbocation − sp2 hybridization, trigonal planar
Carbanion − sp3 hybridization, pyramidal
Free radical − sp3 hybridization, shallow pyramidal geometry. It is so because, due to the presence of single electron in the 2pz orbital, it is not completely pyramidal, however, the electron-electron repulsion forces the bonds to bend from trigonal planar geometry.
Stability −
Inductive Effect − Since this species also lacks octet, it is stabilized by electron donating groups. Thus, greater the number of alkyl groups attached, more is the stability
30 > 20 > 10
Hyperconjugation
More the number of α −hydrogens, more will be the stability of the free radical via hyperconjugation. For example,
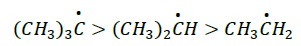
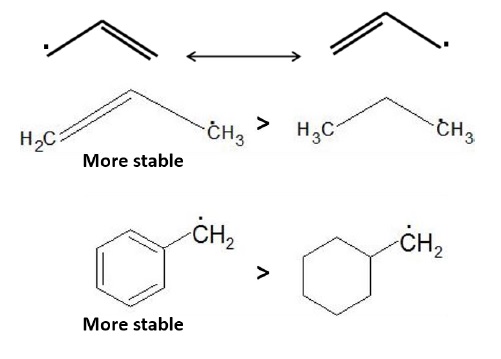
Resonance Effect − charge delocalization also stabilizes electron deficient species. Geometry of free radicals is shallow pyramidal such that the partially filled p −orbital can overlap with adjacent p −orbitals of the π −bonds resulting in delocalization.