Quantitative Analysis - Nitrogen Kjeldahl’s Method I
Description:
Principle
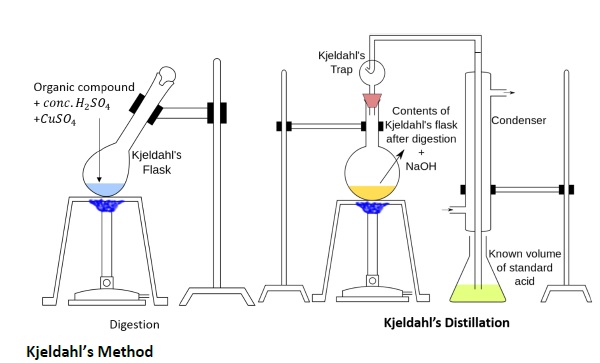
The compound containing nitrogen is heated with concentrated H2SO4.
Nitrogen in the compound gets converted to ammonium sulphate.
The resulting acid mixture is then heated with excess of NaOH
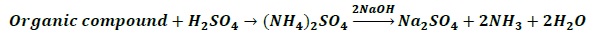
The liberated NH3 gas is absorbed in excess of standard solution of H2SO4.
2NH3 + H2SO4 → (NH4)2SO4
The unreacted or unused acid is estimated by titration with standard alkali solution.
The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
The amount of ammonia produced is estimated by the amount of sulphuric acid that is consumed in the reaction.
Used for the estimation of nitrogen in food, fertilizers and drugs.
Not applicable: to compounds containing nitrogen in the ring (pyridine), and as nitro and diazo groups.
It is because nitrogen in these groups do not change to ammonium sulphate under the above conditions.
Apparatus
Kjeldahl’s digestion flask: where heating of organic compound with conc.H2SO4 is done.
Round bottom flask: with condenser where Kjeldahl’s digestion product is distilled with NaOH.
Titration flask.