Qualitative Analysis - Problem 2
Description:
Which among the given statements is false?
A. Sodium reacts with the elements in an organic compound to form water soluble salts.
B. Lassaigne’s test for nitrogen on H2N − C6H4 − SO3H gives Prussian Blue colour.
C. Sodium cyanide gives positive Lassaigne’s test.
D. Lassaigne’s test for halogens uses HNO3 as Na2S and NaCN are decomposed by HNO3 Solution:
Solution −
The correct option is: (B)
Explanation −
- Option (A): True
Sodium reacts with the elements in an organic compound to convert the covalently bound elements to ionic form and to water soluble salts.
- Option (B): False
Lassaigne’s test for nitrogen on H2N − C6H4 − SO3H gives blood red coloration and not Prussian blue due to the presence of both nitrogen and Sulphur giving SCN- instead CN−ion.
- Option (C): True
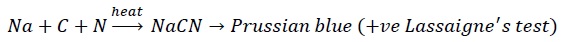
- Option (D): True
In order to remove the interference from other elements such as nitrogen and Sulphur which exist as NaCN and Na2S in the Lassaigne’s extract, conc.HNO3 is added to remove these as HCN and H2S gases.
NaCN + HNO3 → NaNO3 + HCN ↑
Na2S + 2HNO3 → 2NaNO3 + H2S ↑

